MADI Unveils AI-Powered 'Clinical Trial Digital Twin' at CES 2026
MADI Unveils AI-Powered 'Clinical Trial Digital Twin' at CES 2026
Posted January. 06, 2026 10:46,
- MADI, a South Korean AI-CRO startup, unveiled its generative AI-powered clinical trial acceleration platform, MADI-DT, at CES 2026, which simulates patient outcomes and enables pharmaceutical companies to reduce the need for placebo groups, cutting costs and shortening trial timelines.
- The platform is distinguished by its high accuracy for Asian patient characteristics, achieved through training on Korean cohort data, and has been validated by seed funding from major investors.
- MADI aims to address the diversity gap in clinical data and expand its global partnerships, leveraging its foundation of over 40,000 cases of high-fidelity clinical data.
The Seoul Business Agency (SBA), the city's primary support organization for small and medium enterpises (SMEs) led by CEO Hyun-woo Kim, has announced it will operate the "Seoul Integrated Pavilion" at CES 2026 this coming January.
As a dedicated body focused on revitalizing Seoul’s startup ecosystem and identifying high-potential ventures, the SBA is spearheading a massive collaborative effort. A total of 19 Seoul-based organizations, including autonomous district offices, partner institutions, and universities, will join forces to support 70 startups on the global stage.
The Seoul Integrated Pavilion is designed to serve as a strategic springboard for international expansion. Beyond simple exhibition space, the SBA will provide a comprehensive suite of business programs, including on-site investment consultations, buyer matchmaking, global IR pitching sessions, and professional booth operations. Through these initiatives, the agency aims to bridge the gap between local innovation and the global market, ensuring that Seoul’s top startups gain the traction they need for worldwide growth.
MADI, a South Korean AI-CRO (Contract Research Organization) startup, announced its participation in CES 2026, the world’s largest consumer electronics and IT exhibition. Participating as part of the Chung-Ang University delegation at the ‘Seoul Integrated Pavilion,’ MADI will showcase its generative AI-based clinical trial acceleration platform, MADI-DT.
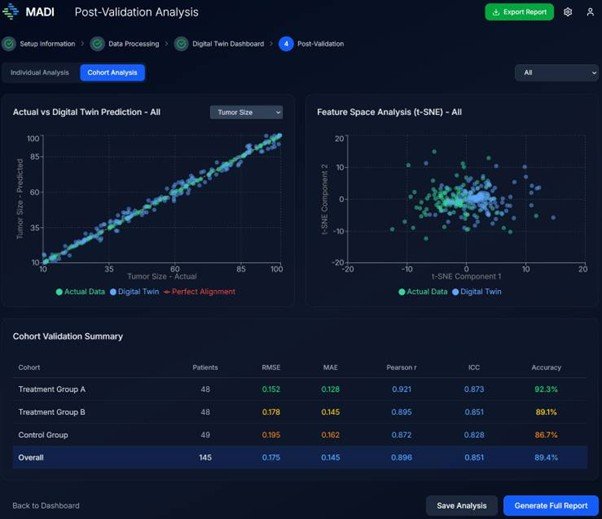
MADI-DT utilizes generative AI to simulate actual patient outcomes, creating "Synthetic Patients." This technology allows pharmaceutical developers to replace essential placebo control groups with virtual counterparts during new drug development. By doing so, MADI estimates it can reduce actual patient recruitment requirements by 20% to 25%. This efficiency translates to an average cost reduction of $2.3 million (approx. 3.3 billion KRW) and can shorten the clinical trial timeline by more than six months.
"Unlike existing competitors that rely heavily on Western-centric data, MADI-DT’s strength lies in its high accuracy optimized for Asian patient characteristics, achieved by training our solution on Korean cohort data," a MADI spokesperson stated.
The spokesperson further emphasized the company's credibility, noting, "Co-founded by medical professors from Chung-Ang University Hospital and AI experts from Seoul National University, MADI has validated its technological prowess by securing seed funding from Samsung Fire & Marine Insurance and Infobank."
Leveraging its presence at CES 2026, MADI plans to solidify its position as a key partner in resolving the industry-wide "Diversity Gap" in clinical data.

Sung-hwan Kim, CEO of MADI and a current professor of Orthopedic Surgery at Chung-Ang University Hospital, highlighted the data-driven foundation of their technology. "MADI has developed its technology based on over 40,000 cases of high-fidelity, time-series clinical data, including Electronic Medical Records (EMR), blood labs, and CT/MRI scans," Kim said.
"Our goal is not merely to assist the clinical process, but to become a premier AI-CRO platform that accelerates the entry of global pharmaceutical companies into the Asian market," Kim added. "Starting with CES, we intend to expand our cooperation with partners in the U.S. and globally."
By Dong-jin Kim (kdj@itdonga.com)
- The platform is distinguished by its high accuracy for Asian patient characteristics, achieved through training on Korean cohort data, and has been validated by seed funding from major investors.
- MADI aims to address the diversity gap in clinical data and expand its global partnerships, leveraging its foundation of over 40,000 cases of high-fidelity clinical data.
The Seoul Business Agency (SBA), the city's primary support organization for small and medium enterpises (SMEs) led by CEO Hyun-woo Kim, has announced it will operate the "Seoul Integrated Pavilion" at CES 2026 this coming January.
As a dedicated body focused on revitalizing Seoul’s startup ecosystem and identifying high-potential ventures, the SBA is spearheading a massive collaborative effort. A total of 19 Seoul-based organizations, including autonomous district offices, partner institutions, and universities, will join forces to support 70 startups on the global stage.
The Seoul Integrated Pavilion is designed to serve as a strategic springboard for international expansion. Beyond simple exhibition space, the SBA will provide a comprehensive suite of business programs, including on-site investment consultations, buyer matchmaking, global IR pitching sessions, and professional booth operations. Through these initiatives, the agency aims to bridge the gap between local innovation and the global market, ensuring that Seoul’s top startups gain the traction they need for worldwide growth.
MADI, a South Korean AI-CRO (Contract Research Organization) startup, announced its participation in CES 2026, the world’s largest consumer electronics and IT exhibition. Participating as part of the Chung-Ang University delegation at the ‘Seoul Integrated Pavilion,’ MADI will showcase its generative AI-based clinical trial acceleration platform, MADI-DT.

MADI-DT, MADI’s proprietary clinical trial acceleration platform / source=MADI
MADI-DT utilizes generative AI to simulate actual patient outcomes, creating "Synthetic Patients." This technology allows pharmaceutical developers to replace essential placebo control groups with virtual counterparts during new drug development. By doing so, MADI estimates it can reduce actual patient recruitment requirements by 20% to 25%. This efficiency translates to an average cost reduction of $2.3 million (approx. 3.3 billion KRW) and can shorten the clinical trial timeline by more than six months.

MADI-DT, MADI’s proprietary clinical trial acceleration platform / source=MADI
"Unlike existing competitors that rely heavily on Western-centric data, MADI-DT’s strength lies in its high accuracy optimized for Asian patient characteristics, achieved by training our solution on Korean cohort data," a MADI spokesperson stated.
The spokesperson further emphasized the company's credibility, noting, "Co-founded by medical professors from Chung-Ang University Hospital and AI experts from Seoul National University, MADI has validated its technological prowess by securing seed funding from Samsung Fire & Marine Insurance and Infobank."
Leveraging its presence at CES 2026, MADI plans to solidify its position as a key partner in resolving the industry-wide "Diversity Gap" in clinical data.

Sung-hwan Kim, CEO of MADI(second from right) with team members / source=MADI
Sung-hwan Kim, CEO of MADI and a current professor of Orthopedic Surgery at Chung-Ang University Hospital, highlighted the data-driven foundation of their technology. "MADI has developed its technology based on over 40,000 cases of high-fidelity, time-series clinical data, including Electronic Medical Records (EMR), blood labs, and CT/MRI scans," Kim said.
"Our goal is not merely to assist the clinical process, but to become a premier AI-CRO platform that accelerates the entry of global pharmaceutical companies into the Asian market," Kim added. "Starting with CES, we intend to expand our cooperation with partners in the U.S. and globally."
By Dong-jin Kim (kdj@itdonga.com)



![[단독]‘아들 주택 11채’ 김경, 공천 보류됐다 강선우가 밀어붙여 구제](https://dimg.donga.com/c/138/175/90/1/wps/NEWS/IMAGE/2026/01/07/133103173.6.jpg)



