FDA recommends approval of Pfizer vaccine
FDA recommends approval of Pfizer vaccine
Posted December. 12, 2020 07:58,
Updated December. 12, 2020 07:58
The U.S. Food and Drug Administration (FDA) was advised on Thursday (local time) by the Vaccines and Related Biological Products Advisory Committee (VRBPAC) or its advisory arm to authorize the urgent use of the COVID-19 vaccine co-developed by U.S. pharmaceutical firm Pfizer and German biotech company BioNtech. In effect, the validation process to determine on vaccination across the United States is coming to an end, even with further decisions by the FDA and the Centers for Disease Control still up in the air. A mass vaccination of U.S. citizens is expected to begin early next week at the earliest.
The VRBPAC’s meeting was held to answer if the efficacy of a vaccination of a person aged 16 or above is greater than the risks. The voting was 17 in favor, four in opposition and one abstention out of a total of 22 commissioners.
Starting from next Monday, at the earliest, vaccines are likely to be provided to the U.S. public on the condition that the CDC expedites a vote on the issue. Health and Human Services Secretary Alex Azar estimated that 20 million U.S. citizens will get inoculated within the next several weeks, adding that between next January and March vaccines will be provided upon their provision.
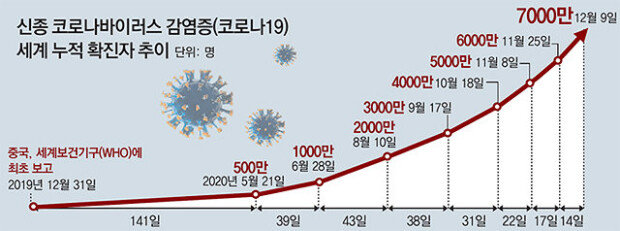
Nevertheless, there is no assurance that the pandemic of COVID-19 will be mitigated in the nation hardest-hit by the virus in the world with 16 million confirmed cases and 300,000 deaths in total. Real-time COVID-19 statistics provider Worldometer announced that as of Wednesday the daily number of U.S. deaths amounted in the aggregate to a record high of 3,263.
jyr0101@donga.com







